Coronary anatomy: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
It has become such a common tool in diagnosing coronary artery disease, that it is hard to understand it’s relatively short history. Radner was the first researcher in 1945 to visualize the coronary arteries in humans, through a transsternal puncture <cite>Radner</cite>. In 1958 Sones at the Cleveland Clinic succeeded in injecting small amounts of contrast material directly in the coronary arteries<cite>Sones</cite>. In the late sixties Judkins developed the percutaneous transfemoral approach and used pre-bent catheters to cannulate the coronary ostia. In the seventies Charles Dotter and Andreas Gruntzig extended the catheterization to therapeutic uses. In the nineties, vascular acces via the radial artery became a realistic alternative. | It has become such a common tool in diagnosing coronary artery disease, that it is hard to understand it’s relatively short history. Radner was the first researcher in 1945 to visualize the coronary arteries in humans, through a transsternal puncture <cite>Radner</cite>. In 1958 Sones at the Cleveland Clinic succeeded in injecting small amounts of contrast material directly in the coronary arteries<cite>Sones</cite>. In the late sixties Judkins developed the percutaneous transfemoral approach and used pre-bent catheters to cannulate the coronary ostia. In the seventies Charles Dotter and Andreas Gruntzig extended the catheterization to therapeutic uses. In the nineties, vascular acces via the radial artery became a realistic alternative. | ||
=Indications and Contra-indications= | |||
Cardiac catheterizations were performed 3 million times a year worldwide in 2010. In the Netherlands around 65.000 catheterizations are performed annually. | Cardiac catheterizations were performed 3 million times a year worldwide in 2010. In the Netherlands around 65.000 catheterizations are performed annually. | ||
| Line 41: | Line 41: | ||
Patients with older age, diabetes mellitus, chronic renal insufficiency, multivessel disease, low ejection fraction have a higher risk of complications. | Patients with older age, diabetes mellitus, chronic renal insufficiency, multivessel disease, low ejection fraction have a higher risk of complications. | ||
=Vascular Access Site= | |||
Before Judkins developed the percutaneous transfemoral approach in the late sixties, brachial arteriotomy was performed to introduce the catheter. This is seldomly used nowadays. In the majority of cases arterial catheters are introduced via the femoral artery or radial artery using the Seldinger technique. | Before Judkins developed the percutaneous transfemoral approach in the late sixties, brachial arteriotomy was performed to introduce the catheter. This is seldomly used nowadays. In the majority of cases arterial catheters are introduced via the femoral artery or radial artery using the Seldinger technique. | ||
==''Technique of access: femoral artery''== | |||
The femoral artery is located just below the inguinal ligament. For the femoral artery access, the femoral head provides the best visible landmark. Arterial puncture at this site remains below the inguinal ligament, is generally above the bifurcation of the superfical femoral and deep femoral arteries, and allows for hemostasis. In obese patients the inguinal skin crease is generally too low. | The femoral artery is located just below the inguinal ligament. For the femoral artery access, the femoral head provides the best visible landmark. Arterial puncture at this site remains below the inguinal ligament, is generally above the bifurcation of the superfical femoral and deep femoral arteries, and allows for hemostasis. In obese patients the inguinal skin crease is generally too low. | ||
| Line 51: | Line 51: | ||
<br/> | <br/> | ||
==''Technique of access: radial artery''== | |||
The catheter is inserted through the preferably the right radial artery which is punctured 1-2 cm proximal to the styloid process after subcutaneous local anesthesia. To prevent arterial spasm a spasmolytic coctail with nitroglycerin (200mcg) and verapamil (5mg) can be administered intravenously<cite>Jukema3</cite>. Repeated arterial trauma increases the risk for spasm. Arterial puncture can be performed with either a single anterior wall puncture or a double wall through-and-through puncture. A small calibre guidewire is inserted through the plastic cannula to facilitate sheath placement. Introducing sheaths are generally 5-French or 6-French, are hydrophilic and have a tapered tip. Catheter advancement is typically performed with a standard 0.035” J-tipped wire, gently advanced until resistance is met. Common causes of resistance are congenital anatomic variations such as the radial artery loop, tortuosity in the axillary, subclavian or inominate artery, and arterial spasm<cite>Caputo</cite>. | The catheter is inserted through the preferably the right radial artery which is punctured 1-2 cm proximal to the styloid process after subcutaneous local anesthesia. To prevent arterial spasm a spasmolytic coctail with nitroglycerin (200mcg) and verapamil (5mg) can be administered intravenously<cite>Jukema3</cite>. Repeated arterial trauma increases the risk for spasm. Arterial puncture can be performed with either a single anterior wall puncture or a double wall through-and-through puncture. A small calibre guidewire is inserted through the plastic cannula to facilitate sheath placement. Introducing sheaths are generally 5-French or 6-French, are hydrophilic and have a tapered tip. Catheter advancement is typically performed with a standard 0.035” J-tipped wire, gently advanced until resistance is met. Common causes of resistance are congenital anatomic variations such as the radial artery loop, tortuosity in the axillary, subclavian or inominate artery, and arterial spasm<cite>Caputo</cite>. | ||
<br/> | <br/> | ||
==''Which route?''== | |||
Complications from arterial access include arterial dissection, AV fistula formation, retroperitoneal hemorrhage and pseudoaneurysma formation. The femoral artery has traditionally been the artery of choice for procedures, but has serious limitations in patients with peripheral vascular disease or using anticoagulation. The radial artery has replaced the femoral artery approach as primary choice in most institutions in Europe. Main advantages are the less severe access site bleedings due to good hemostasis technique by p.e. the TR band, possible earlier mobilization of the patient and lower incidence of local bleeding complications. Disadvantages are the possibility of serious arterial spasm and the risk of radial artery occlusion. | Complications from arterial access include arterial dissection, AV fistula formation, retroperitoneal hemorrhage and pseudoaneurysma formation. The femoral artery has traditionally been the artery of choice for procedures, but has serious limitations in patients with peripheral vascular disease or using anticoagulation. The radial artery has replaced the femoral artery approach as primary choice in most institutions in Europe. Main advantages are the less severe access site bleedings due to good hemostasis technique by p.e. the TR band, possible earlier mobilization of the patient and lower incidence of local bleeding complications. Disadvantages are the possibility of serious arterial spasm and the risk of radial artery occlusion. | ||
<br/> | <br/> | ||
| Line 61: | Line 61: | ||
A randomized trial published in 2008 shower that transradial coronary angiography was safe, feasible and effective with similar results to those of the transfemoral approach. The Access trial performed by Kiemeneij et al showed similar procedural and clinical outcomes of PCI in transradial, transbrachial and transfemoral PCI. Major access site complications were lower in the transradial group, but with higher access failure (coronary cannulation)<cite>Kiemeneij</cite>. Procedural duration and radiation exposure are higher using transradial access, but with significantly lower rate of major vascular complications<cite>Brueck</cite>. In STEMI patients, the HORIZONS-AMI trial showed that the transradial approach was associated with reduced major bleeding and improved event-free survival<cite>Genereux</cite>. | A randomized trial published in 2008 shower that transradial coronary angiography was safe, feasible and effective with similar results to those of the transfemoral approach. The Access trial performed by Kiemeneij et al showed similar procedural and clinical outcomes of PCI in transradial, transbrachial and transfemoral PCI. Major access site complications were lower in the transradial group, but with higher access failure (coronary cannulation)<cite>Kiemeneij</cite>. Procedural duration and radiation exposure are higher using transradial access, but with significantly lower rate of major vascular complications<cite>Brueck</cite>. In STEMI patients, the HORIZONS-AMI trial showed that the transradial approach was associated with reduced major bleeding and improved event-free survival<cite>Genereux</cite>. | ||
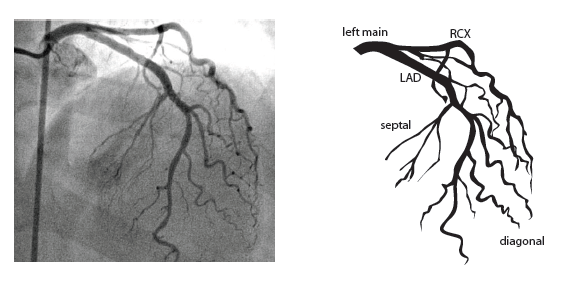
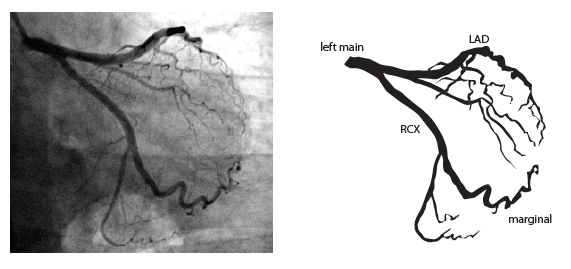
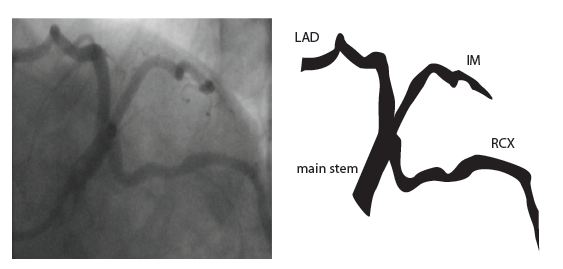
=Coronary anatomy= | |||
[[File:Coronary_anatomy1.png]]<cite>Fauci</cite> | [[File:Coronary_anatomy1.png]]<cite>Fauci</cite> | ||
| Line 72: | Line 71: | ||
The left main coronary artery (LMCA) originated in the left sinus of Valsalva. Its length varies from 5-10mm. Sporadically the LMCA is absent, resulting in separated ostia of RCx and LAD. Sometimes there is a trifurcation, with a branch between the RCx and LAD called intermediate artery. Usually the LAD runs in the anterior interventricular groove. The most important side branches are the septal branches and diagonal branches to the left ventricular wall. The RCx runs in the left atrioventricular groove. All branches to the left ventricular wall are classified as obtuse marginal or posterolateral branches. In 40% the sinus node artery arises from the proximal portion of the RCx. | The left main coronary artery (LMCA) originated in the left sinus of Valsalva. Its length varies from 5-10mm. Sporadically the LMCA is absent, resulting in separated ostia of RCx and LAD. Sometimes there is a trifurcation, with a branch between the RCx and LAD called intermediate artery. Usually the LAD runs in the anterior interventricular groove. The most important side branches are the septal branches and diagonal branches to the left ventricular wall. The RCx runs in the left atrioventricular groove. All branches to the left ventricular wall are classified as obtuse marginal or posterolateral branches. In 40% the sinus node artery arises from the proximal portion of the RCx. | ||
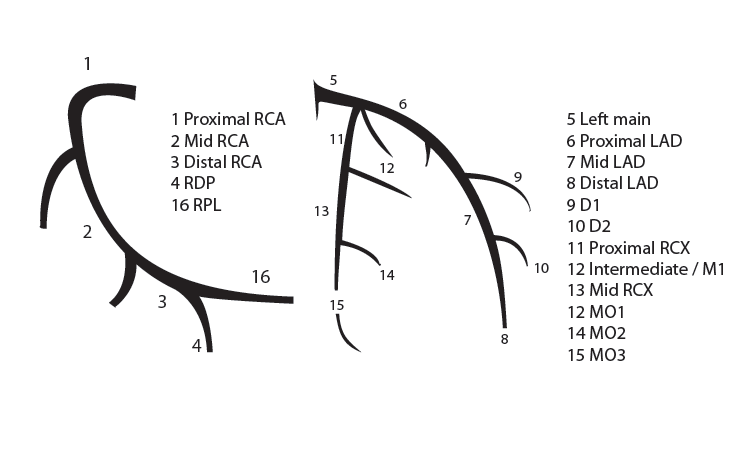
==''Nomenclature of segments''== | |||
[[File:NomenclatureOfSegments.png]] | [[File:NomenclatureOfSegments.png]] | ||
=''Recommended radiographic projections''= | |||
Recommended projections for the RCA are: | Recommended projections for the RCA are: | ||
| Line 105: | Line 104: | ||
Spider view | Spider view | ||
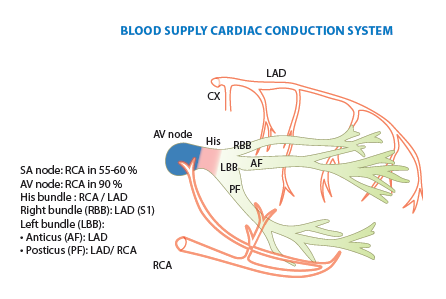
=''Blood supply cardiac conduction system''= | |||
[[File:BloodSupplyCardiacConductionSystem.png]] | [[File:BloodSupplyCardiacConductionSystem.png]] | ||
=''Collateral circulation''= | |||
Collateral connections between coronary arteries are present in every individual<cite>Jukema5</cite>. Because of the higher perfusion pressures in major arteries, there is normally no flow in collateral connections. In case of a coronary obstruction of more than 70% diameter reduction, blood starts to flow to the artery distal of the obstruction. These pre-existent collaterals are called recruitable collaterals. Collaterals can also be newly formed and can be intra- or intercoronary. Cardiac ischemia stimulates formation of new collaterals and growth of recruitable collaterals. Recruitable vessels have a corkscrew aspect. Filling can be retrograde or antegrade (as by bridge collaterals: intracoronary collaterals). Coronary steal means that the flow through the epicardial coronary artery is diminished by the flow to collaterals that it is causing cardiac ischemia. | Collateral connections between coronary arteries are present in every individual<cite>Jukema5</cite>. Because of the higher perfusion pressures in major arteries, there is normally no flow in collateral connections. In case of a coronary obstruction of more than 70% diameter reduction, blood starts to flow to the artery distal of the obstruction. These pre-existent collaterals are called recruitable collaterals. Collaterals can also be newly formed and can be intra- or intercoronary. Cardiac ischemia stimulates formation of new collaterals and growth of recruitable collaterals. Recruitable vessels have a corkscrew aspect. Filling can be retrograde or antegrade (as by bridge collaterals: intracoronary collaterals). Coronary steal means that the flow through the epicardial coronary artery is diminished by the flow to collaterals that it is causing cardiac ischemia. | ||
| Line 117: | Line 116: | ||
= References = | |||
<biblio> | <biblio> | ||
#Radner pmid=19023714 | #Radner pmid=19023714 | ||
Revision as of 20:43, 3 March 2014
Introduction and History
Coronary angiography is a minimally invasive procedure to access the coronary circulation and blood filled chambers of the heart using a catheter. It is performed for both diagnostic and therapeutic purposes.
It has become such a common tool in diagnosing coronary artery disease, that it is hard to understand it’s relatively short history. Radner was the first researcher in 1945 to visualize the coronary arteries in humans, through a transsternal puncture [1]. In 1958 Sones at the Cleveland Clinic succeeded in injecting small amounts of contrast material directly in the coronary arteries[2]. In the late sixties Judkins developed the percutaneous transfemoral approach and used pre-bent catheters to cannulate the coronary ostia. In the seventies Charles Dotter and Andreas Gruntzig extended the catheterization to therapeutic uses. In the nineties, vascular acces via the radial artery became a realistic alternative.
Indications and Contra-indications
Cardiac catheterizations were performed 3 million times a year worldwide in 2010. In the Netherlands around 65.000 catheterizations are performed annually.
These are the indications for cardiac catheterization:
- Class I
- High risk acute coronary syndrome, STEMI acute, NSTEMI within 72 hours, depending risk stratification (p.e. Grace risk score)
- Angina pectoris CCS III/IV despite medical therapy with high risk of ischemia
- After out-of-hospital cardiac arrest (OHCA) with VT/angina pectoris
- Pre-cardiac valve surgery (male > 35 years, female > 50 years)
- Class IIa
- Decreased left ventricular function, stable angina pectoris CCS I-II/IV with objectified ischemia
- Inconclusive or conflicting results after non-invasive stress testing
- Class IIb
- Angina pectoris CCS III/IV with improvement on medication
- Class III
- Angina pectoris CCS I/II, using medication, without objectified ischemia
There are no absolute contra-indications for coronary angiography. Relative contra-indications include:
- Coagulopathy
- Decompensated congestive heart failure
- Uncontrolled hypertension
- Recent CVA
- Refractory arrhytmia
- Renal failure
- Contrast medium allergy
Although coronary angiography, when performed lege artis and for the right indication is a relatively safe procedure, complications do occur in left heart catheterization in 1-2%. Major complications are death (0.1%), myocardial ischemia (0.1%), arrhytmias (0.4%), major bleedings (0.15-2.5%), vascular complications, CVA (0.2%) and contrast reaction. Patients with older age, diabetes mellitus, chronic renal insufficiency, multivessel disease, low ejection fraction have a higher risk of complications.
Vascular Access Site
Before Judkins developed the percutaneous transfemoral approach in the late sixties, brachial arteriotomy was performed to introduce the catheter. This is seldomly used nowadays. In the majority of cases arterial catheters are introduced via the femoral artery or radial artery using the Seldinger technique.
Technique of access: femoral artery
The femoral artery is located just below the inguinal ligament. For the femoral artery access, the femoral head provides the best visible landmark. Arterial puncture at this site remains below the inguinal ligament, is generally above the bifurcation of the superfical femoral and deep femoral arteries, and allows for hemostasis. In obese patients the inguinal skin crease is generally too low.
After subcutaneous local anesthesia, a small incision is made and the needle held in a 45 degrees angle to puncture the front wall of the artery. When pulsating blood is obtained, the flexible tip of the guide wire is inserted and advanced in the aorta to the level of the diaphragm. The needle is removed and an introducer plus sheath is inserted[3].
Technique of access: radial artery
The catheter is inserted through the preferably the right radial artery which is punctured 1-2 cm proximal to the styloid process after subcutaneous local anesthesia. To prevent arterial spasm a spasmolytic coctail with nitroglycerin (200mcg) and verapamil (5mg) can be administered intravenously[4]. Repeated arterial trauma increases the risk for spasm. Arterial puncture can be performed with either a single anterior wall puncture or a double wall through-and-through puncture. A small calibre guidewire is inserted through the plastic cannula to facilitate sheath placement. Introducing sheaths are generally 5-French or 6-French, are hydrophilic and have a tapered tip. Catheter advancement is typically performed with a standard 0.035” J-tipped wire, gently advanced until resistance is met. Common causes of resistance are congenital anatomic variations such as the radial artery loop, tortuosity in the axillary, subclavian or inominate artery, and arterial spasm[5].
Which route?
Complications from arterial access include arterial dissection, AV fistula formation, retroperitoneal hemorrhage and pseudoaneurysma formation. The femoral artery has traditionally been the artery of choice for procedures, but has serious limitations in patients with peripheral vascular disease or using anticoagulation. The radial artery has replaced the femoral artery approach as primary choice in most institutions in Europe. Main advantages are the less severe access site bleedings due to good hemostasis technique by p.e. the TR band, possible earlier mobilization of the patient and lower incidence of local bleeding complications. Disadvantages are the possibility of serious arterial spasm and the risk of radial artery occlusion.
A randomized trial published in 2008 shower that transradial coronary angiography was safe, feasible and effective with similar results to those of the transfemoral approach. The Access trial performed by Kiemeneij et al showed similar procedural and clinical outcomes of PCI in transradial, transbrachial and transfemoral PCI. Major access site complications were lower in the transradial group, but with higher access failure (coronary cannulation)[6]. Procedural duration and radiation exposure are higher using transradial access, but with significantly lower rate of major vascular complications[7]. In STEMI patients, the HORIZONS-AMI trial showed that the transradial approach was associated with reduced major bleeding and improved event-free survival[8].
Coronary anatomy
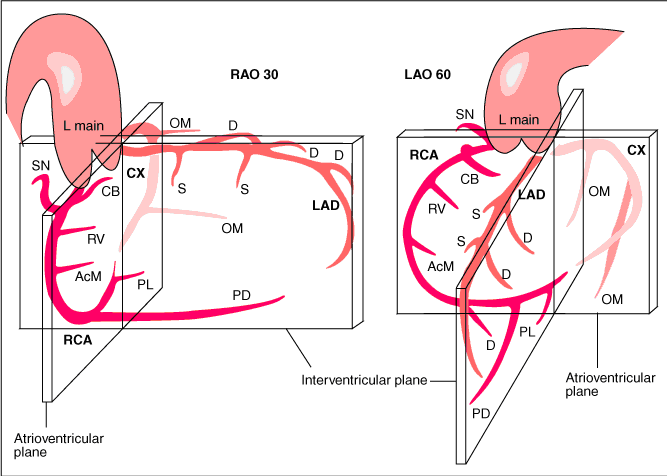 [9]
[9]
The main coronary arteries may be considered to be located in two planes: the plane of the atrioventricular groove and the plane of the interventricular septum[10].
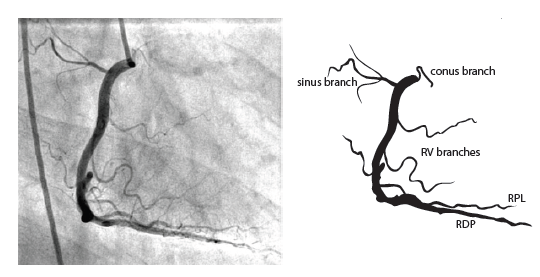
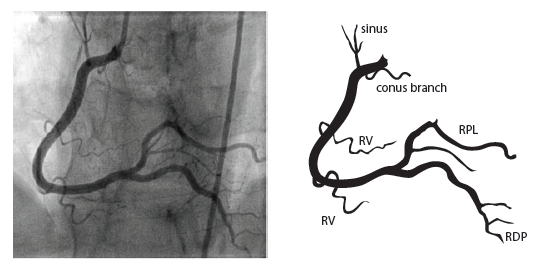
The right coronary artery (RCA) originates in the right sinus of Valsalva and runs in the right ventricular side of the atrioventricular groove. At the crux the posterior descending artery (RDP) and atrioventricular node artery originate. If the RCA continues after the RDP to supply a portion of the posterior left ventricular wall (RPL), it is called a right dominant circulation (85% of people). If the LCA supplies the posterior left ventricular wall (LPL) the coronary circulation is called left dominant (5%), in 10% of people there is balanced system. The RDP runs in the posterior interventricular groove. In 60% the sinus node artery arises from the proximal portion of the RCA.
The left main coronary artery (LMCA) originated in the left sinus of Valsalva. Its length varies from 5-10mm. Sporadically the LMCA is absent, resulting in separated ostia of RCx and LAD. Sometimes there is a trifurcation, with a branch between the RCx and LAD called intermediate artery. Usually the LAD runs in the anterior interventricular groove. The most important side branches are the septal branches and diagonal branches to the left ventricular wall. The RCx runs in the left atrioventricular groove. All branches to the left ventricular wall are classified as obtuse marginal or posterolateral branches. In 40% the sinus node artery arises from the proximal portion of the RCx.
Nomenclature of segments
Recommended radiographic projections
Recommended projections for the RCA are:
- LAO 45 Proximal and mid-RCA
- RAO 30 Mid-RCA, RDPcollateral vessels to LAD
- LAO 20 cranial 25 Crux, RDP and RPL
Recommended projections for the LCA are:
- Cranial 40 (0- RAO 5) Left main and LAD proximal, mid and distal, diagonals
- Caudal 40 (0- RAO 30) Left main, bifurcations, LAD proximal, RCx proximal, mid and distal, MO-bifurcations
- LAO 50 caudal 25 (spider view) Left main, bifurcations LAD and RCx
Blood supply cardiac conduction system
Collateral circulation
Collateral connections between coronary arteries are present in every individual[11]. Because of the higher perfusion pressures in major arteries, there is normally no flow in collateral connections. In case of a coronary obstruction of more than 70% diameter reduction, blood starts to flow to the artery distal of the obstruction. These pre-existent collaterals are called recruitable collaterals. Collaterals can also be newly formed and can be intra- or intercoronary. Cardiac ischemia stimulates formation of new collaterals and growth of recruitable collaterals. Recruitable vessels have a corkscrew aspect. Filling can be retrograde or antegrade (as by bridge collaterals: intracoronary collaterals). Coronary steal means that the flow through the epicardial coronary artery is diminished by the flow to collaterals that it is causing cardiac ischemia.
For collateral connections, it is necessary to make longer angiographic projections. In a RCA obstruction, intercoronary collaterals can form between the septal branches of the LAD and the RDP through the interventricular septum. Collaterals connecting distal portions of two arteries are frequently observed, p.e. connections between the distal RCx and RCA in the interventricular groove and between diagonal branches of the LAD. A collateral between the conus branch of the RCA to the proximal LAD is called a ring of Vieussens. Atrial branches from the RCA or the Kugel’s artery (mostly an small artery arising from proximal RCA anastomosing with branches of sinus node artery) can form connections between the proximal and distal RCA.
References
- Radner S. An attempt at the roentgenologic visualization of coronary blood vessels in man. Acta Radiol Suppl (Stockholm). 2008 Aug;434:43-6. DOI:10.1080/02841850802133345 |
- SONES FM Jr and SHIREY EK. Cine coronary arteriography. Mod Concepts Cardiovasc Dis. 1962 Jul;31:735-8.
-
Jukema2 JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 2: 10.
-
JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 2: 10.
- Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, and Patel T. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011 Nov 15;78(6):823-39. DOI:10.1002/ccd.23052 |
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, and van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997 May;29(6):1269-75. DOI:10.1016/s0735-1097(97)00064-8 |
- Brueck M, Bandorski D, Kramer W, Wieczorek M, Höltgen R, and Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009 Nov;2(11):1047-54. DOI:10.1016/j.jcin.2009.07.016 |
- Généreux P, Mehran R, Palmerini T, Caixeta A, Kirtane AJ, Lansky AJ, Brodie BR, Witzenbichler B, Mockel M, Guagliumi G, Peruga JZ, Dudek D, Fahy MP, Dangas G, Stone GW, and HORIZONS-AMI Trial Investigators. Radial access in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty in acute myocardial infarction: the HORIZONS-AMI trial. EuroIntervention. 2011 Dec;7(8):905-16. DOI:10.4244/EIJV7I8A144 |
-
Fauci AS, Kasper DL, Braunwald E, Hauser SL, Longo DL. Harrison’s Principles of Internal Medicine, 17th edition: http://www.accessmedicine.com
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 3: 23-34.
-
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 3: 23-34.
-
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 4: 35-40.
-
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 1: 7