Coronary anatomy: Difference between revisions
>Martijnmeuwissen No edit summary |
|||
| (39 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
* [[ | =Introduction and History= | ||
* [[Left | |||
Coronary angiography is a minimally invasive procedure to access the coronary circulation and blood filled chambers of the heart using a catheter. It is performed for both diagnostic and therapeutic purposes. | |||
It has become such a common tool in diagnosing coronary artery disease, that it is hard to understand it’s relatively short history. Radner was the first researcher in 1945 to visualize the coronary arteries in humans, through a transsternal puncture <cite>Radner</cite>. In 1958 Sones at the Cleveland Clinic succeeded in injecting small amounts of contrast material directly in the coronary arteries<cite>Sones</cite>. In the late sixties Judkins developed the percutaneous transfemoral approach and used pre-bent catheters to cannulate the coronary ostia. In the seventies Charles Dotter and Andreas Gruntzig extended the catheterization to therapeutic uses. In the nineties, vascular acces via the radial artery became a realistic alternative. | |||
=Indications and Contra-indications= | |||
Cardiac catheterizations were performed 3 million times a year worldwide in 2010. In the Netherlands around 65.000 catheterizations are performed annually. | |||
These are the indications for cardiac catheterization: | |||
*Class I | |||
- High risk acute coronary syndrome, STEMI acute, NSTEMI within 72 hours, depending risk stratification (p.e. Grace risk score) | |||
<br/> | |||
- Angina pectoris CCS III/IV despite medical therapy with high risk of ischemia | |||
<br/> | |||
- After out-of-hospital cardiac arrest (OHCA) with VT/angina pectoris | |||
<br/> | |||
- Pre-cardiac valve surgery (male > 35 years, female > 50 years) | |||
<br/> | |||
*Class IIa | |||
- Decreased left ventricular function, stable angina pectoris CCS I-II/IV with objectified ischemia | |||
<br/> | |||
- Inconclusive or conflicting results after non-invasive stress testing | |||
<br/> | |||
*Class IIb | |||
- Angina pectoris CCS III/IV with improvement on medication | |||
<br/> | |||
*Class III | |||
- Angina pectoris CCS I/II, using medication, without objectified ischemia | |||
<br/> | |||
There are no absolute contra-indications for coronary angiography. Relative contra-indications include: | |||
*Coagulopathy | |||
*Decompensated congestive heart failure | |||
*Uncontrolled hypertension | |||
*Recent CVA | |||
*Refractory arrhytmia | |||
*Renal failure | |||
*Contrast medium allergy | |||
Although coronary angiography, when performed lege artis and for the right indication is a relatively safe procedure, complications do occur in left heart catheterization in 1-2%. Major complications are death (0.1%), myocardial ischemia (0.1%), arrhytmias (0.4%), major bleedings (0.15-2.5%), vascular complications, CVA (0.2%) and contrast reaction. | |||
Patients with older age, diabetes mellitus, chronic renal insufficiency, multivessel disease, low ejection fraction have a higher risk of complications. | |||
=Time-out procedure= | |||
In surgery, the use of a pre-operative checklist has improved the outcome. Implementation of a 19-item surgical safety checklist improved team communication and reduced rates of death and complications in patients undergoing noncardiac surgery<cite>Haynes</cite>. Implementation of a comprehensive preoperative checklist targeting the entire surgical pathway (including items such as medication, marking of operative side and the use of postoperatieve instructions) was associated with a reduction in surgical complications and mortality in hospitals with a high standard of care<cite>deVries</cite>. | |||
Although the goal of most procedures in interventional cardiology is to access the heart and its associated vasculature (making wrong site procedures less of a concern), a preprocedure checklist is also recommended in the catheterization laboratory<cite>Naidu</cite>. Information obtained preprocedural should include procedural indication, patients history, informed consent, a review of medication (in particular antiplatelet therapies and metformin) and a risk of bleeding assessment. Renal function should be less than 90 days old. If the patient is using VKA an INR is obtained < 24 preprocedural. Images of prior catheterizations are obtained and procedural reports of any coronary or peripheral bypass surgery reviewed. The history should be reviewed for previous heparin-induced thrombocytopenia (HIT). Allergies should be checked, especially contrast allergies or allergies to medication used periprocedural (p.e. heparin). Each laboratory has a protocol for preventing contrast allergic reaction (using p.e. Prednison and an antihistaminic). | |||
Before procedure a time-out procedure is performed by all team members before vascular access is obtained. Patient identification should be checked and confirmed and agreement on the right procedure obtained. Figure 1 shows a sample of a time-out checklist. | |||
Figure 1 | |||
<br/> | |||
Sample ‘‘Time Out’’ Preprocedure Checklist | |||
The physician taking ultimate responsibility for the procedure should lead the Time Out and ensure each of the following items is announced: | |||
#Patient’s name and medical record number | |||
#Procedure to be performed (e.g., left heart catheterization, coronary angiography, right heart catheterization) | |||
#Route to be used (e.g., right femoral artery) | |||
#Confirm that the equipment needed is available or alternatives are available including intended stent type for PCI or cath-possible patients | |||
#Patient’s allergies and premedication if appropriate (e.g., heparin-induced thrombocytopenia, contrast allergy) | |||
#Special laboratory or medical conditions (e.g., elevated INR, chronic kidney disease) | |||
=Vascular Access Site= | |||
Before Judkins developed the percutaneous transfemoral approach in the late sixties, brachial arteriotomy was performed to introduce the catheter. This is seldomly used nowadays. In the majority of cases arterial catheters are introduced via the femoral artery or radial artery using the Seldinger technique. | |||
==Technique of access: femoral artery== | |||
The femoral artery is located just below the inguinal ligament. For the femoral artery access, the femoral head provides the best visible landmark. Arterial puncture at this site remains below the inguinal ligament, is generally above the bifurcation of the superfical femoral and deep femoral arteries, and allows for hemostasis. In obese patients the inguinal skin crease is generally too low. | |||
After subcutaneous local anesthesia, a small incision is made and the needle held in a 45 degrees angle to puncture the front wall of the artery. When pulsating blood is obtained, the flexible tip of the guide wire is inserted and advanced in the aorta to the level of the diaphragm. The needle is removed and an introducer plus sheath is inserted<cite>Jukema2</cite>. | |||
<br/> | |||
==Technique of access: radial artery== | |||
The catheter is inserted through the preferably the right radial artery which is punctured 1-2 cm proximal to the styloid process after subcutaneous local anesthesia. To prevent arterial spasm a spasmolytic coctail with nitroglycerin (200mcg) and verapamil (5mg) can be administered intravenously<cite>Jukema3</cite>. Repeated arterial trauma increases the risk for spasm. Arterial puncture can be performed with either a single anterior wall puncture or a double wall through-and-through puncture. A small calibre guidewire is inserted through the plastic cannula to facilitate sheath placement. Introducing sheaths are generally 5-French or 6-French, are hydrophilic and have a tapered tip. Catheter advancement is typically performed with a standard 0.035” J-tipped wire, gently advanced until resistance is met. Common causes of resistance are congenital anatomic variations such as the radial artery loop, tortuosity in the axillary, subclavian or inominate artery, and arterial spasm<cite>Caputo</cite>. | |||
<br/> | |||
==Which route?== | |||
Complications from arterial access include arterial dissection, AV fistula formation, retroperitoneal hemorrhage and pseudoaneurysma formation. The femoral artery has traditionally been the artery of choice for procedures, but has serious limitations in patients with peripheral vascular disease or using anticoagulation. The radial artery has replaced the femoral artery approach as primary choice in most institutions in Europe. Main advantages are the less severe access site bleedings due to good hemostasis technique by p.e. the TR band, possible earlier mobilization of the patient and lower incidence of local bleeding complications. Disadvantages are the possibility of serious arterial spasm and the risk of radial artery occlusion. | |||
<br/> | |||
A randomized trial published in 2008 shower that transradial coronary angiography was safe, feasible and effective with similar results to those of the transfemoral approach. The Access trial performed by Kiemeneij et al showed similar procedural and clinical outcomes of PCI in transradial, transbrachial and transfemoral PCI. Major access site complications were lower in the transradial group, but with higher access failure (coronary cannulation)<cite>Kiemeneij</cite>. Procedural duration and radiation exposure are higher using transradial access, but with significantly lower rate of major vascular complications<cite>Brueck</cite>. In STEMI patients, the HORIZONS-AMI trial showed that the transradial approach was associated with reduced major bleeding and improved event-free survival<cite>Genereux</cite>. | |||
=Coronary anatomy= | |||
[[File:Coronary_anatomy1.png]]<cite>Fauci</cite> | |||
The main coronary arteries may be considered to be located in two planes: the plane of the atrioventricular groove and the plane of the interventricular septum<cite>Jukema4</cite>. | |||
The right coronary artery (RCA) originates in the right sinus of Valsalva and runs in the right ventricular side of the atrioventricular groove. At the crux the posterior descending artery (RDP) and atrioventricular node artery originate. If the RCA continues after the RDP to supply a portion of the posterior left ventricular wall (RPL), it is called a right dominant circulation (85% of people). If the LCA supplies the posterior left ventricular wall (LPL) the coronary circulation is called left dominant (5%), in 10% of people there is balanced system. The RDP runs in the posterior interventricular groove. In 60% the sinus node artery arises from the proximal portion of the RCA. | |||
The left main coronary artery (LMCA) originated in the left sinus of Valsalva. Its length varies from 5-10mm. Sporadically the LMCA is absent, resulting in separated ostia of RCx and LAD. Sometimes there is a trifurcation, with a branch between the RCx and LAD called intermediate artery. Usually the LAD runs in the anterior interventricular groove. The most important side branches are the septal branches and diagonal branches to the left ventricular wall. The RCx runs in the left atrioventricular groove. All branches to the left ventricular wall are classified as obtuse marginal or posterolateral branches. In 40% the sinus node artery arises from the proximal portion of the RCx. | |||
==Nomenclature of segments== | |||
[[File:NomenclatureOfSegments.png]] | |||
=Recommended radiographic projections= | |||
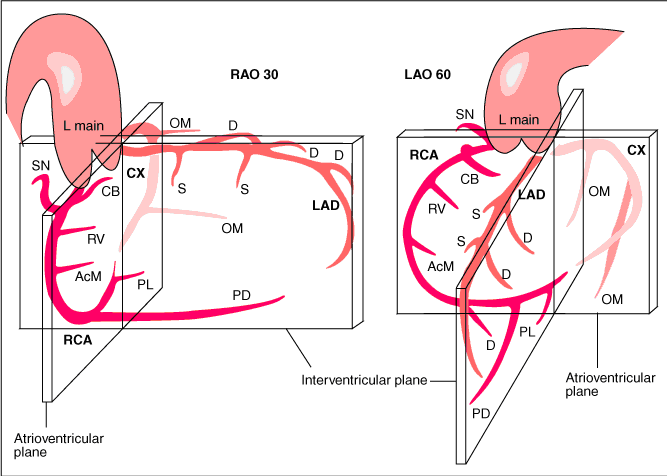
Recommended projections for the RCA are: | |||
*LAO 45 Proximal and mid-RCA | |||
*RAO 30 Mid-RCA, RDPcollateral vessels to LAD | |||
*LAO 20 cranial 25 Crux, RDP and RPL | |||
[[File:RecommendedRadiographicProjections_03072014.png]] | |||
LAO 45 | |||
[[File:RecommendedRadiographicProjections2.png]] | |||
RAO 30 | |||
[[File:RecommendedRadiographicProjections3.png]] | |||
LAO 20 cranial 25 | |||
Recommended projections for the LCA are: | |||
*Cranial 40 (0- RAO 5) Left main and LAD proximal, mid and distal, diagonals | |||
*Caudal 40 (0- RAO 30) Left main, bifurcations, LAD proximal, RCx proximal, mid and distal, MO-bifurcations | |||
*LAO 50 caudal 25 (spider view) Left main, bifurcations LAD and RCx | |||
[[File:RecommendedProjectionsForTheLCA.png]] | |||
Cranial 40 (0-RAO 5) | |||
[[File:RecommendedProjectionsForTheLCA2.png]] | |||
Caudal 40 (0-RAO 30) | |||
[[File:RecommendedProjectionsForTheLCA3.png]] | |||
Spider view | |||
=Blood supply cardiac conduction system= | |||
[[File:BloodSupplyCardiacConductionSystem.png]] | |||
=Collateral circulation= | |||
Collateral connections between coronary arteries are present in every individual<cite>Jukema5</cite>. Because of the higher perfusion pressures in major arteries, there is normally no flow in collateral connections. In case of a coronary obstruction of more than 70% diameter reduction, blood starts to flow to the artery distal of the obstruction. These pre-existent collaterals are called recruitable collaterals. Collaterals can also be newly formed and can be intra- or intercoronary. Cardiac ischemia stimulates formation of new collaterals and growth of recruitable collaterals. Recruitable vessels have a corkscrew aspect. Filling can be retrograde or antegrade (as by bridge collaterals: intracoronary collaterals). Coronary steal means that the flow through the epicardial coronary artery is diminished by the flow to collaterals that it is causing cardiac ischemia. | |||
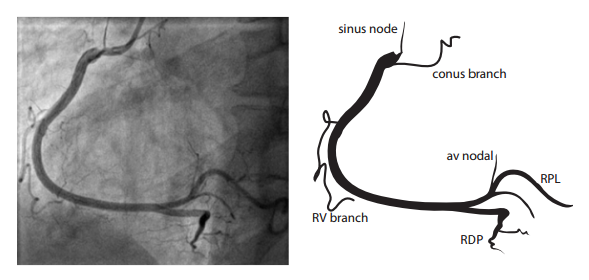
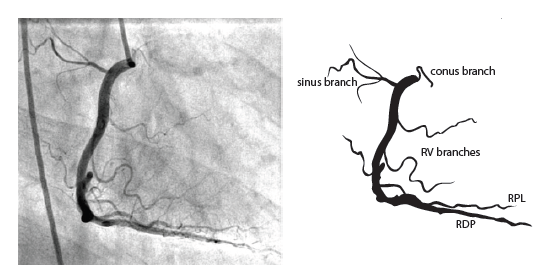
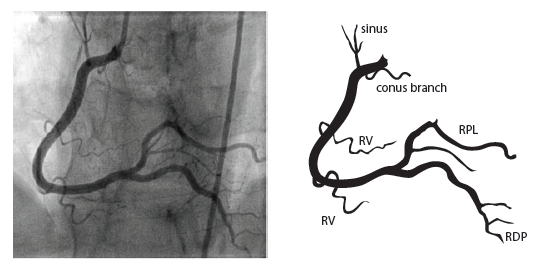
For collateral connections, it is necessary to make longer angiographic projections. In a RCA obstruction, intercoronary collaterals can form between the septal branches of the LAD and the RDP through the interventricular septum. Collaterals connecting distal portions of two arteries are frequently observed, p.e. connections between the distal RCx and RCA in the interventricular groove and between diagonal branches of the LAD. A collateral between the conus branch of the RCA to the proximal LAD is called a ring of Vieussens. Atrial branches from the RCA or the Kugel’s artery (mostly an small artery arising from proximal RCA anastomosing with branches of sinus node artery) can form connections between the proximal and distal RCA. | |||
=Left ventriculography= | |||
Left ventriculography provides information about global and segmental left ventricular function and mitral regurgitation, and some other abnormalities (ventricular septal defect, hypertrophic cardiomyopathy, left ventricular thrombi)<cite>Baim</cite>. | |||
''Technique'' | |||
<br/> | |||
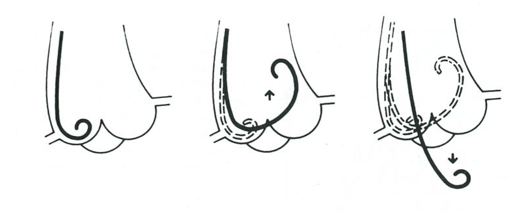
The large amount of contrast that is needed in short time, is delivered by a pigtail catheter with multiple side holes. The RAO 30° projection is used without magnification. The catheter is placed on the aortic valve with a guidewire, retracted a few centimeters in the catheters. After pushing the pigtail on the valve until bending, it is retracted slowly while being rotated clockwise, untill passing into the left ventricle (figure 1<cite>Hartcatheterisatie</cite>). The optimal catheter position is midcavitary. About 35 ml of contrast is delivered at 10ml/second at a PSI of maximal 1000 at RAO 30° and LAO 60°. Left ventriculography can be performed with normal quiet breathing (in LAO, the diaphragm can be obstructive during expiration). | |||
''Interpretation'' | |||
<br/> | |||
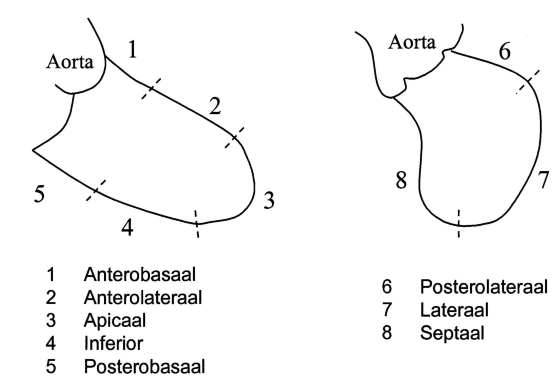
The left ventriculogram is analysed qualitatively on a sinus beat following a previous sinus beat since ectopic of postectopic beats give a false impression of ventricular function. Overall ventricular function is described. In RAO view, the anterolateral, apical, inferior and posterobasal segment can be seen, in LAO view the posterolateral, lateral and septal segments (figure 1). The degree of mitral regurgitation can be estimated. | |||
''Complications'' | |||
<br/> | |||
It is normal for the patient to experience a hot feeling due to powerful vasodilatation of the contrast. Ventricular extrasystoles occur frequently but also ventricular tachycardia or ventricular fibrillation can occur. The pigtail can be placed under chordae (catheter close to inferior wall). If it is placed under the papillary muscles with a side hole firmly against the endocardium, deposition of contrast material within the endocardium and myocardium can occur, leading rarely to myocardial perforation. A possible complication that can lead to death is the injection of air (air embolism). If the catheter is placed in the left ventricular outflow tract, a left anterior fascicular block can occur, leading to complete heart block in pre-existing right bundle branch block and left posterior fascicular block. | |||
<br/> | |||
Figure 1 | |||
<br/> | |||
[[File:TimeOut_figure1.png]] | |||
Figure 2 | |||
<br/> | |||
[[File:TimeOut_figure2.gif]] | |||
#Anterobasal | |||
#Anterolateral | |||
#Apical | |||
#Inferior | |||
#Posterobasal | |||
#Posterolateral | |||
#Lateral | |||
#Septal | |||
Figure 3 | |||
<br/> | |||
[[File:TimeOut_figure3.gif]] | |||
Left ventriculography during systole showing apical ballooning akinesis with basal hyperkinesis in a patient with takotsubo cardiomyopathy. | |||
=Shock= | |||
Cardiogenic shock is the inability of the heart, as a result of impairment of its pumping function, to deliver sufficient blood flow to the tissues to meet resting metabolic demands<cite>Califf</cite>. There is poor cardiac output and evidence of tissue hypoxia in the presence of adequate intravascular volume. The diagnosis is indicated by low blood pressure (<90mm Hg, or <30mm Hg below basal levels), a depressed cardiac index (< 2.2 L/min) in the presence of an elevated pulmonary-capillary wedge pressure (> 15 mmHg). | |||
Causes of cardiogenic shock are: | |||
*Acute myocardial infarction (left or right) | |||
*Mechanical complication | |||
**Acute mitral regurgitation due to papillary muscle dysfunction or rupture | |||
**Ventricular septal rupture | |||
**Free-wall rupture | |||
**Left ventricular aneurysm | |||
*Other causes | |||
**Myocarditis | |||
**Mycoardial contusion | |||
**Left ventricular outflow tract obstruction (aortic stenosis, hypetrophic obstructive cardiomyopathy) | |||
**Left ventricular inflow tract obstruction (mitral stenosis, intracardiac tumour) | |||
**After cardiopulmonary bypass | |||
=Intra-aortic balloon counterpulsation= | |||
''Background'' | |||
<br/> | |||
The intra-aortic balloon pump (IABP) was first introduced in 1968<cite>Kantrowitz</cite>. It uses counterpulsation to increase aortic pressure during diastole and decreasing aortic pressure during systole. This improves the artery blood supply while decreasing the impedance for blood from the ventricle during systole<cite>Baim2</cite>. | |||
[[File:IntraAortic_figure1.jpg]] | |||
<br/> | |||
Figure 1: intra-aortic location of IABP | |||
[[File:IntraAortic_figure2.jpg]] | |||
<br/> | |||
Figure 2: balloon pressure curve. | |||
''Technique'' | |||
<br/> | |||
The balloon pump system consists of a balloon-tipped catheter (polyurethane, 10cm, 40ml, dual lumen) that is positioned in the descending aorta 1 to 2 cm beyond the origin of the left subclavian artery and is inserted percuteaneously through a 8-9 French sheath. The balloon is inflated in with helium after aortic valve closure (triggered on the R wave of surface ECG), and maintained until just before the beginning of systolic ejection when the helium is abruptly withdrawn. When appropriately timed, the effect of the IABP is to reduce ventricular afterload and increase cardiac output. | |||
''Indication'' | |||
<br/> | |||
Indication for IABP: | |||
*Cardiogenic shock secondary to myocardial infarction with continuing ischemia, ventricular septal rupture or mitral regurgitation, myocarditis, sepsis (IIb indication in ESC STEMI guideline<cite>EurHeart</cite>) | |||
*Inability to wean from bypass after cardiac surgery | |||
*Severe arrhythmia owing to refractory ischemia | |||
*Prophylactic high risk intervention (high risk PCI due to LV dysfunction or large territory at risk, severe multivessel disease, left main disease) | |||
Contra-indications are: | |||
*Significant aortic regurgitation | |||
*Abdominal aortic aneurysm | |||
*Aortic dissection | |||
*High risk of bleeding | |||
*Bilateral femoral-popliteal bypass grafts | |||
''Precautions'' | |||
<br/> | |||
The level of anticoagulation should be monitored daily, with aPTT maintained 50-70 seconds to prevent thrombotic or embolic complications. Evalutions of the limbs and peripheral circulation should be checked regularly. Before removal, patients are weaned by decreasing the counterpulsation (to 1:2/1:3). The incidence of major complications is 2.8%. Common complications are limb ischemia, access site bleeding and balloon leak. Risk factors for complications are female sex, older age (>75 years), BSA < 1.65m2 and peripheral vascular disease. | |||
''Clinical results'' | |||
<br/> | |||
In one meta-analyses including 7 randomized trials with STEMI patients with cardiogenic shock, IABP showed neither a 30-day survival benefit nor improved left ventricular ejection fraction, and was associatied with more complications (higher stroke, higher bleeding rates). Another meta-analysis included 9 cohorts with STEMI patients with cardiogenic shock, and showed that IABP was associated with an 18% decrease in 30 day mortality, but with significantly higher revascularization rates and higher mortality in the PCI group<cite>EurHeart2</cite>. In the randomized controlled trial Shock II, publiced in the NEJM in 2012, the use of IABP did not significantly reduce the 30-day mortality in patients with cardiogenic shock complicating acute myocardial infarction for whom an early revascularization strategy was planned<cite>Thiele</cite>. | |||
= Other circulatory assist devices: Impella = | |||
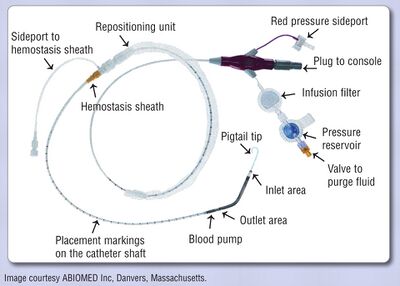
The Impella device is an axial flow pump on a catheter. The system has an electromagnetic motor directy coupled to a helical impeller located near the tip of a catheter. When the “snorkel” at the tip of the catheter is placed across the aortic valve and into the left ventricular chamber, the motor rotates the impeller at 50.000 rpm, drawing ventricular blood into the distal end of the catheter and discharging it non-pulsatile into the ascending aorta. The device can be placed via the femoral artery in a 9 Fr sheath. There are 2 versions: a 2.5 and a 5L (33.000 rpm), with maximal outputs of respectively 2.5 and 5.0L/min. Before placement an echocardiography should rule out LV thrombus<cite>Engstrom</cite>. | |||
Possible indications for Impella placement are high risk PCI and cardiogenic shock. | |||
In 2012, the PROTECT II trial randomized 452 patients with 3-vessel of left main coronary artery disease and severely depressed left ventricular function to an Impella (2.5L) or an IABP. The Impella provided superior hemodynamic support, but with no difference in the 30 days endpoint of major adverse events<cite>Cardiol</cite>. | |||
In 2008, a multicenter registry showed the Impella 2.5 to be safe and potentially useful in hemodynamic support in high-risk PCI (multivessel, left main, last remaining vessel, low LV function) with rates of myocardial infarction, stroke, bleeding and vascular complications at 30 days of 6.2%<cite>Oneill</cite>. | |||
[[File:Impella.jpg|thumb|400px|Example of a 2.5L Impella system<cite>McCulloch</cite>]] | |||
<br/> | |||
= References = | |||
<biblio> | |||
#Radner pmid=19023714 | |||
#Sones pmid=13915182 | |||
#Jukema Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 1: 7 | |||
#Jukema2 Jukema2 JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 2: 10. | |||
#Jukema3 JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 2: 10. | |||
#Caputo pmid=21544927 | |||
#Kiemeneij pmid=9137223 | |||
#Brueck pmid=19926042 | |||
#Genereux pmid=22157475 | |||
#Fauci Fauci AS, Kasper DL, Braunwald E, Hauser SL, Longo DL. Harrison’s Principles of Internal Medicine, 17th edition: http://www.accessmedicine.com | |||
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 3: 23-34. | |||
#Jukema4 Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 3: 23-34. | |||
#Jukema5 Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 4: 35-40. | |||
#Haynes pmid=19144931 | |||
#deVries pmid=21067384 | |||
#Naidu pmid=22434598 | |||
#Baim Baim DS. Grossman’s cardiac catheterization, angiography, and intervention. 7th edition 2006. Lipincott Williams & Wilkins, Philadelphia PA USA. Chapter 12: 222-233. | |||
#Hartcatheterisatie https://www.mst.nl/opleidingcardiologie/modules/hartcatheterisatie/ | |||
#Califf pmid=8190135 | |||
#Kantrowitz pmid=5694059 | |||
#Baim2 Baim DS. Grossman’s cardiac catheterization, angiography, and intervention. 7th edition 2006. Lipincott Williams & Wilkins, Philadelphia PA USA. Chapter 21: 412-430. | |||
#EurHeart pmid=22922416 | |||
#EurHeart2 pmid=19168529 | |||
#Thiele pmid=22920912 | |||
#Engstrom pmid=21118589 | |||
#Cardiol pmid=20082934 | |||
#Oneill pmid=22935569 | |||
#McCulloch pmid=21285459 | |||
</biblio> | |||
Latest revision as of 19:21, 11 December 2016
Introduction and History
Coronary angiography is a minimally invasive procedure to access the coronary circulation and blood filled chambers of the heart using a catheter. It is performed for both diagnostic and therapeutic purposes.
It has become such a common tool in diagnosing coronary artery disease, that it is hard to understand it’s relatively short history. Radner was the first researcher in 1945 to visualize the coronary arteries in humans, through a transsternal puncture [1]. In 1958 Sones at the Cleveland Clinic succeeded in injecting small amounts of contrast material directly in the coronary arteries[2]. In the late sixties Judkins developed the percutaneous transfemoral approach and used pre-bent catheters to cannulate the coronary ostia. In the seventies Charles Dotter and Andreas Gruntzig extended the catheterization to therapeutic uses. In the nineties, vascular acces via the radial artery became a realistic alternative.
Indications and Contra-indications
Cardiac catheterizations were performed 3 million times a year worldwide in 2010. In the Netherlands around 65.000 catheterizations are performed annually.
These are the indications for cardiac catheterization:
- Class I
- High risk acute coronary syndrome, STEMI acute, NSTEMI within 72 hours, depending risk stratification (p.e. Grace risk score)
- Angina pectoris CCS III/IV despite medical therapy with high risk of ischemia
- After out-of-hospital cardiac arrest (OHCA) with VT/angina pectoris
- Pre-cardiac valve surgery (male > 35 years, female > 50 years)
- Class IIa
- Decreased left ventricular function, stable angina pectoris CCS I-II/IV with objectified ischemia
- Inconclusive or conflicting results after non-invasive stress testing
- Class IIb
- Angina pectoris CCS III/IV with improvement on medication
- Class III
- Angina pectoris CCS I/II, using medication, without objectified ischemia
There are no absolute contra-indications for coronary angiography. Relative contra-indications include:
- Coagulopathy
- Decompensated congestive heart failure
- Uncontrolled hypertension
- Recent CVA
- Refractory arrhytmia
- Renal failure
- Contrast medium allergy
Although coronary angiography, when performed lege artis and for the right indication is a relatively safe procedure, complications do occur in left heart catheterization in 1-2%. Major complications are death (0.1%), myocardial ischemia (0.1%), arrhytmias (0.4%), major bleedings (0.15-2.5%), vascular complications, CVA (0.2%) and contrast reaction. Patients with older age, diabetes mellitus, chronic renal insufficiency, multivessel disease, low ejection fraction have a higher risk of complications.
Time-out procedure
In surgery, the use of a pre-operative checklist has improved the outcome. Implementation of a 19-item surgical safety checklist improved team communication and reduced rates of death and complications in patients undergoing noncardiac surgery[3]. Implementation of a comprehensive preoperative checklist targeting the entire surgical pathway (including items such as medication, marking of operative side and the use of postoperatieve instructions) was associated with a reduction in surgical complications and mortality in hospitals with a high standard of care[4].
Although the goal of most procedures in interventional cardiology is to access the heart and its associated vasculature (making wrong site procedures less of a concern), a preprocedure checklist is also recommended in the catheterization laboratory[5]. Information obtained preprocedural should include procedural indication, patients history, informed consent, a review of medication (in particular antiplatelet therapies and metformin) and a risk of bleeding assessment. Renal function should be less than 90 days old. If the patient is using VKA an INR is obtained < 24 preprocedural. Images of prior catheterizations are obtained and procedural reports of any coronary or peripheral bypass surgery reviewed. The history should be reviewed for previous heparin-induced thrombocytopenia (HIT). Allergies should be checked, especially contrast allergies or allergies to medication used periprocedural (p.e. heparin). Each laboratory has a protocol for preventing contrast allergic reaction (using p.e. Prednison and an antihistaminic).
Before procedure a time-out procedure is performed by all team members before vascular access is obtained. Patient identification should be checked and confirmed and agreement on the right procedure obtained. Figure 1 shows a sample of a time-out checklist.
Figure 1
Sample ‘‘Time Out’’ Preprocedure Checklist
The physician taking ultimate responsibility for the procedure should lead the Time Out and ensure each of the following items is announced:
- Patient’s name and medical record number
- Procedure to be performed (e.g., left heart catheterization, coronary angiography, right heart catheterization)
- Route to be used (e.g., right femoral artery)
- Confirm that the equipment needed is available or alternatives are available including intended stent type for PCI or cath-possible patients
- Patient’s allergies and premedication if appropriate (e.g., heparin-induced thrombocytopenia, contrast allergy)
- Special laboratory or medical conditions (e.g., elevated INR, chronic kidney disease)
Vascular Access Site
Before Judkins developed the percutaneous transfemoral approach in the late sixties, brachial arteriotomy was performed to introduce the catheter. This is seldomly used nowadays. In the majority of cases arterial catheters are introduced via the femoral artery or radial artery using the Seldinger technique.
Technique of access: femoral artery
The femoral artery is located just below the inguinal ligament. For the femoral artery access, the femoral head provides the best visible landmark. Arterial puncture at this site remains below the inguinal ligament, is generally above the bifurcation of the superfical femoral and deep femoral arteries, and allows for hemostasis. In obese patients the inguinal skin crease is generally too low.
After subcutaneous local anesthesia, a small incision is made and the needle held in a 45 degrees angle to puncture the front wall of the artery. When pulsating blood is obtained, the flexible tip of the guide wire is inserted and advanced in the aorta to the level of the diaphragm. The needle is removed and an introducer plus sheath is inserted[6].
Technique of access: radial artery
The catheter is inserted through the preferably the right radial artery which is punctured 1-2 cm proximal to the styloid process after subcutaneous local anesthesia. To prevent arterial spasm a spasmolytic coctail with nitroglycerin (200mcg) and verapamil (5mg) can be administered intravenously[7]. Repeated arterial trauma increases the risk for spasm. Arterial puncture can be performed with either a single anterior wall puncture or a double wall through-and-through puncture. A small calibre guidewire is inserted through the plastic cannula to facilitate sheath placement. Introducing sheaths are generally 5-French or 6-French, are hydrophilic and have a tapered tip. Catheter advancement is typically performed with a standard 0.035” J-tipped wire, gently advanced until resistance is met. Common causes of resistance are congenital anatomic variations such as the radial artery loop, tortuosity in the axillary, subclavian or inominate artery, and arterial spasm[8].
Which route?
Complications from arterial access include arterial dissection, AV fistula formation, retroperitoneal hemorrhage and pseudoaneurysma formation. The femoral artery has traditionally been the artery of choice for procedures, but has serious limitations in patients with peripheral vascular disease or using anticoagulation. The radial artery has replaced the femoral artery approach as primary choice in most institutions in Europe. Main advantages are the less severe access site bleedings due to good hemostasis technique by p.e. the TR band, possible earlier mobilization of the patient and lower incidence of local bleeding complications. Disadvantages are the possibility of serious arterial spasm and the risk of radial artery occlusion.
A randomized trial published in 2008 shower that transradial coronary angiography was safe, feasible and effective with similar results to those of the transfemoral approach. The Access trial performed by Kiemeneij et al showed similar procedural and clinical outcomes of PCI in transradial, transbrachial and transfemoral PCI. Major access site complications were lower in the transradial group, but with higher access failure (coronary cannulation)[9]. Procedural duration and radiation exposure are higher using transradial access, but with significantly lower rate of major vascular complications[10]. In STEMI patients, the HORIZONS-AMI trial showed that the transradial approach was associated with reduced major bleeding and improved event-free survival[11].
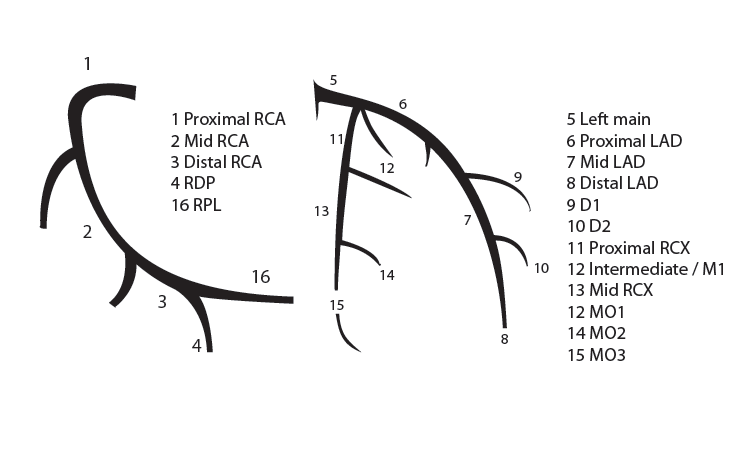
Coronary anatomy
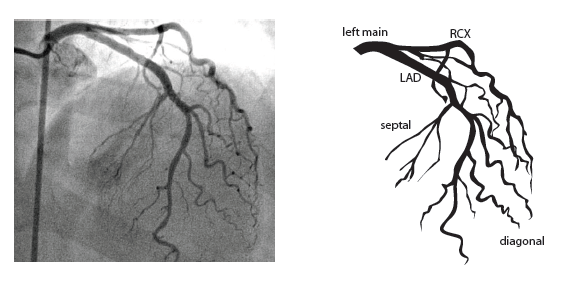
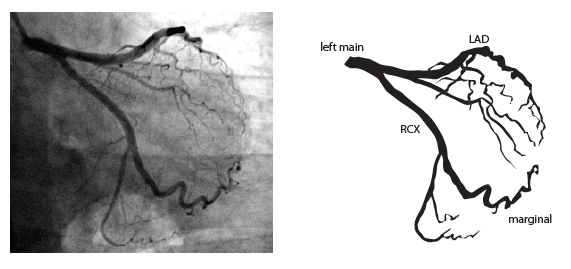
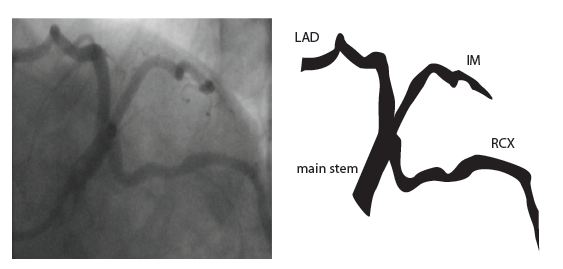
 [12]
[12]
The main coronary arteries may be considered to be located in two planes: the plane of the atrioventricular groove and the plane of the interventricular septum[13].
The right coronary artery (RCA) originates in the right sinus of Valsalva and runs in the right ventricular side of the atrioventricular groove. At the crux the posterior descending artery (RDP) and atrioventricular node artery originate. If the RCA continues after the RDP to supply a portion of the posterior left ventricular wall (RPL), it is called a right dominant circulation (85% of people). If the LCA supplies the posterior left ventricular wall (LPL) the coronary circulation is called left dominant (5%), in 10% of people there is balanced system. The RDP runs in the posterior interventricular groove. In 60% the sinus node artery arises from the proximal portion of the RCA.
The left main coronary artery (LMCA) originated in the left sinus of Valsalva. Its length varies from 5-10mm. Sporadically the LMCA is absent, resulting in separated ostia of RCx and LAD. Sometimes there is a trifurcation, with a branch between the RCx and LAD called intermediate artery. Usually the LAD runs in the anterior interventricular groove. The most important side branches are the septal branches and diagonal branches to the left ventricular wall. The RCx runs in the left atrioventricular groove. All branches to the left ventricular wall are classified as obtuse marginal or posterolateral branches. In 40% the sinus node artery arises from the proximal portion of the RCx.
Nomenclature of segments
Recommended radiographic projections
Recommended projections for the RCA are:
- LAO 45 Proximal and mid-RCA
- RAO 30 Mid-RCA, RDPcollateral vessels to LAD
- LAO 20 cranial 25 Crux, RDP and RPL
Recommended projections for the LCA are:
- Cranial 40 (0- RAO 5) Left main and LAD proximal, mid and distal, diagonals
- Caudal 40 (0- RAO 30) Left main, bifurcations, LAD proximal, RCx proximal, mid and distal, MO-bifurcations
- LAO 50 caudal 25 (spider view) Left main, bifurcations LAD and RCx
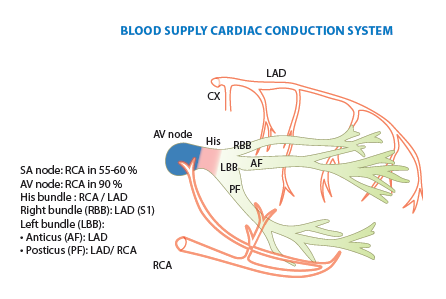
Blood supply cardiac conduction system
Collateral circulation
Collateral connections between coronary arteries are present in every individual[14]. Because of the higher perfusion pressures in major arteries, there is normally no flow in collateral connections. In case of a coronary obstruction of more than 70% diameter reduction, blood starts to flow to the artery distal of the obstruction. These pre-existent collaterals are called recruitable collaterals. Collaterals can also be newly formed and can be intra- or intercoronary. Cardiac ischemia stimulates formation of new collaterals and growth of recruitable collaterals. Recruitable vessels have a corkscrew aspect. Filling can be retrograde or antegrade (as by bridge collaterals: intracoronary collaterals). Coronary steal means that the flow through the epicardial coronary artery is diminished by the flow to collaterals that it is causing cardiac ischemia.
For collateral connections, it is necessary to make longer angiographic projections. In a RCA obstruction, intercoronary collaterals can form between the septal branches of the LAD and the RDP through the interventricular septum. Collaterals connecting distal portions of two arteries are frequently observed, p.e. connections between the distal RCx and RCA in the interventricular groove and between diagonal branches of the LAD. A collateral between the conus branch of the RCA to the proximal LAD is called a ring of Vieussens. Atrial branches from the RCA or the Kugel’s artery (mostly an small artery arising from proximal RCA anastomosing with branches of sinus node artery) can form connections between the proximal and distal RCA.
Left ventriculography
Left ventriculography provides information about global and segmental left ventricular function and mitral regurgitation, and some other abnormalities (ventricular septal defect, hypertrophic cardiomyopathy, left ventricular thrombi)[15].
Technique
The large amount of contrast that is needed in short time, is delivered by a pigtail catheter with multiple side holes. The RAO 30° projection is used without magnification. The catheter is placed on the aortic valve with a guidewire, retracted a few centimeters in the catheters. After pushing the pigtail on the valve until bending, it is retracted slowly while being rotated clockwise, untill passing into the left ventricle (figure 1[16]). The optimal catheter position is midcavitary. About 35 ml of contrast is delivered at 10ml/second at a PSI of maximal 1000 at RAO 30° and LAO 60°. Left ventriculography can be performed with normal quiet breathing (in LAO, the diaphragm can be obstructive during expiration).
Interpretation
The left ventriculogram is analysed qualitatively on a sinus beat following a previous sinus beat since ectopic of postectopic beats give a false impression of ventricular function. Overall ventricular function is described. In RAO view, the anterolateral, apical, inferior and posterobasal segment can be seen, in LAO view the posterolateral, lateral and septal segments (figure 1). The degree of mitral regurgitation can be estimated.
Complications
It is normal for the patient to experience a hot feeling due to powerful vasodilatation of the contrast. Ventricular extrasystoles occur frequently but also ventricular tachycardia or ventricular fibrillation can occur. The pigtail can be placed under chordae (catheter close to inferior wall). If it is placed under the papillary muscles with a side hole firmly against the endocardium, deposition of contrast material within the endocardium and myocardium can occur, leading rarely to myocardial perforation. A possible complication that can lead to death is the injection of air (air embolism). If the catheter is placed in the left ventricular outflow tract, a left anterior fascicular block can occur, leading to complete heart block in pre-existing right bundle branch block and left posterior fascicular block.
- Anterobasal
- Anterolateral
- Apical
- Inferior
- Posterobasal
- Posterolateral
- Lateral
- Septal
Figure 3
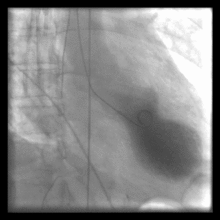 Left ventriculography during systole showing apical ballooning akinesis with basal hyperkinesis in a patient with takotsubo cardiomyopathy.
Left ventriculography during systole showing apical ballooning akinesis with basal hyperkinesis in a patient with takotsubo cardiomyopathy.
Shock
Cardiogenic shock is the inability of the heart, as a result of impairment of its pumping function, to deliver sufficient blood flow to the tissues to meet resting metabolic demands[17]. There is poor cardiac output and evidence of tissue hypoxia in the presence of adequate intravascular volume. The diagnosis is indicated by low blood pressure (<90mm Hg, or <30mm Hg below basal levels), a depressed cardiac index (< 2.2 L/min) in the presence of an elevated pulmonary-capillary wedge pressure (> 15 mmHg).
Causes of cardiogenic shock are:
- Acute myocardial infarction (left or right)
- Mechanical complication
- Acute mitral regurgitation due to papillary muscle dysfunction or rupture
- Ventricular septal rupture
- Free-wall rupture
- Left ventricular aneurysm
- Other causes
- Myocarditis
- Mycoardial contusion
- Left ventricular outflow tract obstruction (aortic stenosis, hypetrophic obstructive cardiomyopathy)
- Left ventricular inflow tract obstruction (mitral stenosis, intracardiac tumour)
- After cardiopulmonary bypass
Intra-aortic balloon counterpulsation
Background
The intra-aortic balloon pump (IABP) was first introduced in 1968[18]. It uses counterpulsation to increase aortic pressure during diastole and decreasing aortic pressure during systole. This improves the artery blood supply while decreasing the impedance for blood from the ventricle during systole[19].
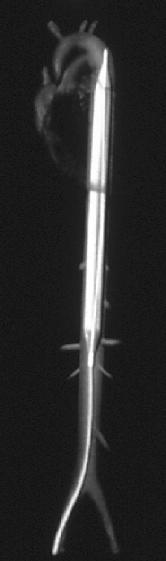
Figure 1: intra-aortic location of IABP
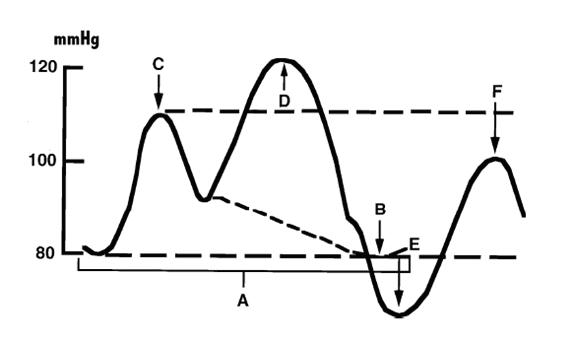
Figure 2: balloon pressure curve.
Technique
The balloon pump system consists of a balloon-tipped catheter (polyurethane, 10cm, 40ml, dual lumen) that is positioned in the descending aorta 1 to 2 cm beyond the origin of the left subclavian artery and is inserted percuteaneously through a 8-9 French sheath. The balloon is inflated in with helium after aortic valve closure (triggered on the R wave of surface ECG), and maintained until just before the beginning of systolic ejection when the helium is abruptly withdrawn. When appropriately timed, the effect of the IABP is to reduce ventricular afterload and increase cardiac output.
Indication
Indication for IABP:
- Cardiogenic shock secondary to myocardial infarction with continuing ischemia, ventricular septal rupture or mitral regurgitation, myocarditis, sepsis (IIb indication in ESC STEMI guideline[20])
- Inability to wean from bypass after cardiac surgery
- Severe arrhythmia owing to refractory ischemia
- Prophylactic high risk intervention (high risk PCI due to LV dysfunction or large territory at risk, severe multivessel disease, left main disease)
Contra-indications are:
- Significant aortic regurgitation
- Abdominal aortic aneurysm
- Aortic dissection
- High risk of bleeding
- Bilateral femoral-popliteal bypass grafts
Precautions
The level of anticoagulation should be monitored daily, with aPTT maintained 50-70 seconds to prevent thrombotic or embolic complications. Evalutions of the limbs and peripheral circulation should be checked regularly. Before removal, patients are weaned by decreasing the counterpulsation (to 1:2/1:3). The incidence of major complications is 2.8%. Common complications are limb ischemia, access site bleeding and balloon leak. Risk factors for complications are female sex, older age (>75 years), BSA < 1.65m2 and peripheral vascular disease.
Clinical results
In one meta-analyses including 7 randomized trials with STEMI patients with cardiogenic shock, IABP showed neither a 30-day survival benefit nor improved left ventricular ejection fraction, and was associatied with more complications (higher stroke, higher bleeding rates). Another meta-analysis included 9 cohorts with STEMI patients with cardiogenic shock, and showed that IABP was associated with an 18% decrease in 30 day mortality, but with significantly higher revascularization rates and higher mortality in the PCI group[21]. In the randomized controlled trial Shock II, publiced in the NEJM in 2012, the use of IABP did not significantly reduce the 30-day mortality in patients with cardiogenic shock complicating acute myocardial infarction for whom an early revascularization strategy was planned[22].
Other circulatory assist devices: Impella
The Impella device is an axial flow pump on a catheter. The system has an electromagnetic motor directy coupled to a helical impeller located near the tip of a catheter. When the “snorkel” at the tip of the catheter is placed across the aortic valve and into the left ventricular chamber, the motor rotates the impeller at 50.000 rpm, drawing ventricular blood into the distal end of the catheter and discharging it non-pulsatile into the ascending aorta. The device can be placed via the femoral artery in a 9 Fr sheath. There are 2 versions: a 2.5 and a 5L (33.000 rpm), with maximal outputs of respectively 2.5 and 5.0L/min. Before placement an echocardiography should rule out LV thrombus[23].
Possible indications for Impella placement are high risk PCI and cardiogenic shock.
In 2012, the PROTECT II trial randomized 452 patients with 3-vessel of left main coronary artery disease and severely depressed left ventricular function to an Impella (2.5L) or an IABP. The Impella provided superior hemodynamic support, but with no difference in the 30 days endpoint of major adverse events[24]. In 2008, a multicenter registry showed the Impella 2.5 to be safe and potentially useful in hemodynamic support in high-risk PCI (multivessel, left main, last remaining vessel, low LV function) with rates of myocardial infarction, stroke, bleeding and vascular complications at 30 days of 6.2%[25].
References
- Radner S. An attempt at the roentgenologic visualization of coronary blood vessels in man. Acta Radiol Suppl (Stockholm). 2008 Aug;434:43-6. DOI:10.1080/02841850802133345 |
- SONES FM Jr and SHIREY EK. Cine coronary arteriography. Mod Concepts Cardiovasc Dis. 1962 Jul;31:735-8.
- Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, Herbosa T, Joseph S, Kibatala PL, Lapitan MC, Merry AF, Moorthy K, Reznick RK, Taylor B, Gawande AA, and Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009 Jan 29;360(5):491-9. DOI:10.1056/NEJMsa0810119 |
- de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, van Helden SH, Schlack WS, van Putten MA, Gouma DJ, Dijkgraaf MG, Smorenburg SM, Boermeester MA, and SURPASS Collaborative Group. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010 Nov 11;363(20):1928-37. DOI:10.1056/NEJMsa0911535 |
- Naidu SS, Rao SV, Blankenship J, Cavendish JJ, Farah T, Moussa I, Rihal CS, Srinivas VS, Yakubov SJ, and Society for Cardiovascular Angiography and Interventions. Clinical expert consensus statement on best practices in the cardiac catheterization laboratory: Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012 Sep 1;80(3):456-64. DOI:10.1002/ccd.24311 |
-
Jukema2 JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 2: 10.
-
JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 2: 10.
- Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, and Patel T. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011 Nov 15;78(6):823-39. DOI:10.1002/ccd.23052 |
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, and van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997 May;29(6):1269-75. DOI:10.1016/s0735-1097(97)00064-8 |
- Brueck M, Bandorski D, Kramer W, Wieczorek M, Höltgen R, and Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009 Nov;2(11):1047-54. DOI:10.1016/j.jcin.2009.07.016 |
- Généreux P, Mehran R, Palmerini T, Caixeta A, Kirtane AJ, Lansky AJ, Brodie BR, Witzenbichler B, Mockel M, Guagliumi G, Peruga JZ, Dudek D, Fahy MP, Dangas G, Stone GW, and HORIZONS-AMI Trial Investigators. Radial access in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty in acute myocardial infarction: the HORIZONS-AMI trial. EuroIntervention. 2011 Dec;7(8):905-16. DOI:10.4244/EIJV7I8A144 |
-
Fauci AS, Kasper DL, Braunwald E, Hauser SL, Longo DL. Harrison’s Principles of Internal Medicine, 17th edition: http://www.accessmedicine.com
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 3: 23-34.
-
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 3: 23-34.
-
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 4: 35-40.
-
Baim DS. Grossman’s cardiac catheterization, angiography, and intervention. 7th edition 2006. Lipincott Williams & Wilkins, Philadelphia PA USA. Chapter 12: 222-233.
- Califf RM and Bengtson JR. Cardiogenic shock. N Engl J Med. 1994 Jun 16;330(24):1724-30. DOI:10.1056/NEJM199406163302406 |
- Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, and Sherman JL Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968 Jan 8;203(2):113-8.
-
Baim DS. Grossman’s cardiac catheterization, angiography, and intervention. 7th edition 2006. Lipincott Williams & Wilkins, Philadelphia PA USA. Chapter 21: 412-430.
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, and Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012 Oct;33(20):2569-619. DOI:10.1093/eurheartj/ehs215 |
- Sjauw KD, Engström AE, Vis MM, van der Schaaf RJ, Baan J Jr, Koch KT, de Winter RJ, Piek JJ, Tijssen JG, and Henriques JP. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines?. Eur Heart J. 2009 Feb;30(4):459-68. DOI:10.1093/eurheartj/ehn602 |
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K, and IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012 Oct 4;367(14):1287-96. DOI:10.1056/NEJMoa1208410 |
- Engström AE, de Mol BA, Lagrand WK, and Henriques JP. [The Impella system for mechanical support and myocardial recovery]. Ned Tijdschr Geneeskd. 2010;154(45):A2699.
- Sjauw KD, Konorza T, Erbel R, Danna PL, Viecca M, Minden HH, Butter C, Engstrøm T, Hassager C, Machado FP, Pedrazzini G, Wagner DR, Schamberger R, Kerber S, Mathey DG, Schofer J, Engström AE, and Henriques JP. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009 Dec 15;54(25):2430-4. DOI:10.1016/j.jacc.2009.09.018 |
- O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Dzavík V, Popma J, Douglas PS, and Ohman M. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012 Oct 2;126(14):1717-27. DOI:10.1161/CIRCULATIONAHA.112.098194 |
- McCulloch B. Use of the Impella 2.5 in high-risk percutaneous coronary intervention. Crit Care Nurse. 2011 Feb;31(1):e1-16. DOI:10.4037/ccn2011293 |
-
Jukema JW, Vliegen HW, Bruschke AVG. Coronary angiography: principles, technique and interpretation. 1e druk, Leiden, the Netherlands, 2009. Chapter 1: 7